Fanshawe lab developments will help speed the validation process for COVID-19 treatments
 CREDIT: NICOLAS LORAN
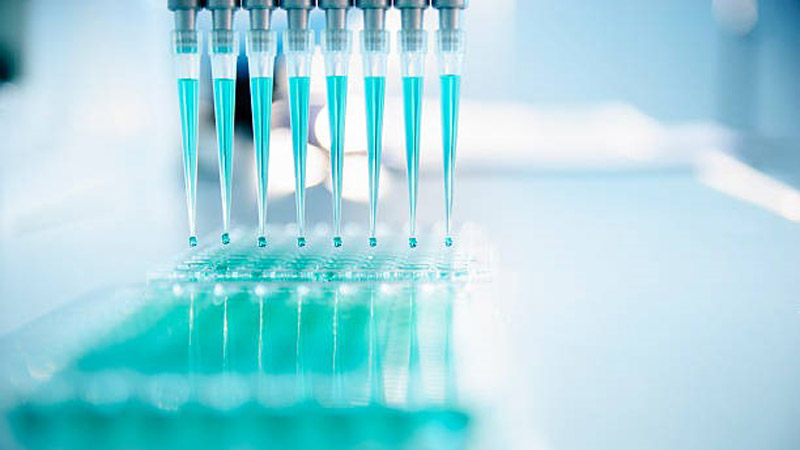
CREDIT: NICOLAS LORANUsing high throughput screening, pharmaceutical companies are able to test up to 96 different drugs for effectiveness.
Fanshawe College’s Centre for Research and Innovation has developed a faster and cheaper method of testing potential treatments for COVID-19.
Principal Investigator Abdulla Mahboob has developed copies of the SARS-COV-2 virus called “replicons.” These replicons contain the genetic material of the virus but lack the instructions that allow it to infect other cells. This means the replicons themselves are non-infectious, which means they don’t require the use of a biosafety level-2 laboratory.
Rather than use the genetic instructions to infect other cells, Mahboob said that what they have done is use genetic material from fireflies that encodes for luminescence.
“We put the gene into the genome of the replicon, and what that means is every time the virus makes a copy of itself, you have more and more luminescence,” explained Mahboob.
This means if you were to test the replicon against a potential drug treatment, less luminescence would mean the drug was working.
While Mahboob said that the use of replicons is not new, the system that his team has created is one that is easy to manipulate.
“If you are trying to test a new kind of drug against resistant forms of the virus…you can, and you can do so very safely without the need of a biosafety lab,” he said.
Fanshawe’s replicon has been developed with two problematic mutations of COVID-19: one that is associated with higher mortality and another that is resistant against the current treatment remdesivir.
Another way the replicons will help speed up testing is that with this method, pharmaceutical companies would not have to measure the viral RNA (the amount of viral genetic material), they would just need to measure for the luminescence. Pharmaceutical companies often have thousands of drugs at their disposal that can now be tested at a faster rate using something called high throughput screening.
“You have a plate and on that plate you have many, many wells,” said Mahboob. “And in each well you can imagine a different treatment and you can then measure the luminescence of them all at the same time.”
This could mean that 96 different treatments could be effectively tested at the same time.
“You don’t need to have any sophisticated equipment to measure the viral genetic material because you don’t need that,” said Mahboob. “Every time the virus makes a copy, you make more luminescence.”
Chair of Research, Colin Yates, said the next step for this development is to find a commercial partner to take it to a larger scale application.
“We’re still looking for partners,” he said. “We’re actively engaging and pursuing various partnerships to help us get it past the proof of concept and into the prototype and clinical stage.”
Ultimately, these developments could mean that people seeking treatment for COVID-19 could potentially get the drugs they need faster, especially as the virus changes over time.
“As the virus mutates and evolves, as all viruses do, we can more rapidly figure out potential treatments,” said Yates. “Rather than by the time you figured out the new treatment, it’s often mutated again. You’re always one step behind, so hopefully this helps speed up that process.”
“The faster we can effectively test the latest treatment options, the better our chances of potentially saving lives,” added Mahboob.

















